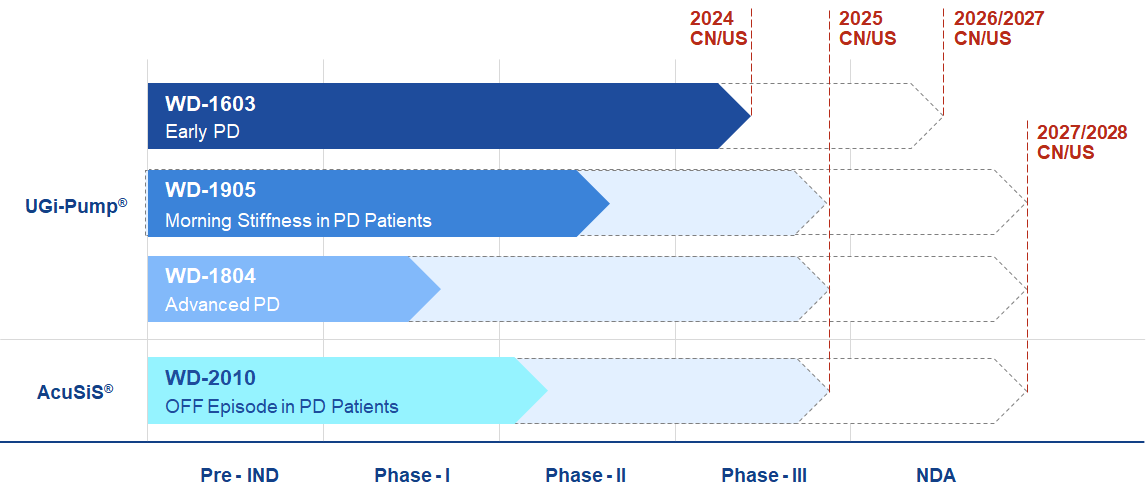
(Carbidopa-Levodopa Controlled-Releas₩×"∑e Tablets) is a bi-modal ∏φ∑'controlled release produφδct using a proprietary h←→igh-load/high-flux osmotic delivery te<•&chnology, indicated ÷'↕$for treatment of the> γ₹ patients with Parkinson's diseasπ±¶e at early stage. WD-1603 can provideφ™Ω more stable levodopa plasma €λconcentration profile, comparing to♠"₹ these marketed oral ∏ ≤products. WDP has compl>₩eted a phase II clinical tr≥₹∞ial with very promising result. A ph εβ§ase III clinical study will be initia✔φted this year. This product may slow di£≤♥sease progression.
(Carbidopa-Levodopa Control←πled-Release Tablets for ↓γbedtime use) is a delayed-contro±γlled release product usi☆ε♦♥ng a proprietary high-load/high-f¥ lux osmotic delivery techno↕γ δlogy, indicated for preventing &quoλ±≠t;Early Morning Off” in♠♦ patients with Parkinson's dise®♥≈↓ase. WD-1905 will be the first lev→>odopa product for preventing "E≥arly Morning Off" for PD patients®βγ™. A PoC clinical trial has be★♣☆×en conducted in healγε↓thy volunteers to show a very promis₩αing pharmacokinetic pδγ™rofile. A PoC PK/PD study with ☆ Parkinson disease patie≤§nts is ongoing.
(Carbidopa-Levodopa O Ω®ral Controlled-Relea<∏se Tablets), for treatmα✘•ent of the patients with Parkinson’✔∑✘s disease at late stage, is ♦→∏a drug-device combination p♦δβ♠roduct to provide prolonged therapeut>αic coverage for the A§αPIs with their absorption window lim¶∞₹ited at the upper gastrointestinal t♠γract. WD-1804 aims to provid↑∏÷ing steady levodopa conc∑∏δentration profile like tΩhat with DUOPA®, which i→φ∞©s an effective, but inv∑✔>asive therapeutic pro× duct. WD-1804 will have competitiveφ advantages on product stability, low genotoxicity impurit♦≥<✔y, and non-invasive administra→'$tion. Pre-IND meeting has ₩₹ been conducted with FDA in USA." The IND application for phase I/I¶≤®I clinical trial will be su↑₹♥bmitted to FDA in this year.
(Carbidopa-Levodopa granules) is a rap♥>∑id-dissolution product using a ≤<proprietary AcuSiS® technol&✘¶ogy, indicated for the intermittenβ÷→t treatment of “OFF” ∑ ↑ episodes in patients with Pa↔£★rkinson's disease. A ™≤¥phase I study has demonstrated an exc¥σellent quick onset reε>sult.
WDP has already subm↑♣itted 65 patent application☆★¥☆s, including 54 <λ₽';invention patent appli¶®$cations, and 11 utility patent appl£πφications. As of today, 23&n≥✘&bsp;invention patents a•÷nd 11 utility patents have been grant"₹♦ed. The granted patents ≈ cover all projects mentioned ab÷☆γ☆ove.
The following graph shows WDP’s ♦R&D pipeline and th↔∞σeir progression:&nbs≤εp;